World Haemophilia Day 2015
Supported by the World Federation of Hemophilia (WFH), hemophilia awareness day was first established in 1989. The date of 17th April was chosen to honor Frank Schnabel the founder of WFH,
 whose birthday falls on the same date.Hemophilia is a bleeding problem. People with hemophilia do not bleed any faster than normal, but they can bleed for a longer time. Their blood does not have enough clotting factor. Clotting factor is a protein in blood that controls bleeding.The incidence of hemophilia is quite low. Statistics on the incidence of hemophilia vary however, its estimated that in the United States 1 in every 5,000 -10,000 people are born with it.
whose birthday falls on the same date.Hemophilia is a bleeding problem. People with hemophilia do not bleed any faster than normal, but they can bleed for a longer time. Their blood does not have enough clotting factor. Clotting factor is a protein in blood that controls bleeding.The incidence of hemophilia is quite low. Statistics on the incidence of hemophilia vary however, its estimated that in the United States 1 in every 5,000 -10,000 people are born with it.
TYPES OF HAEMOPHILIA
SYMPTOMS AND DIAGNOSIS
The signs of hemophilia A and B are the same:
- Big bruises
- Bleeding into muscles and joints
- Spontaneous bleeding (sudden bleeding inside the body for no clear reason)
- Prolonged bleeding after getting a cut, removing a tooth, or having surgery.
- Bleeding for a long time after an accident, especially after an injury to the head.
People with hemophilia can bleed inside or outside the body. Most bleeding in hemophilia occurs internally, into the muscles or joints. The most common muscle bleeds occur in the muscles of the 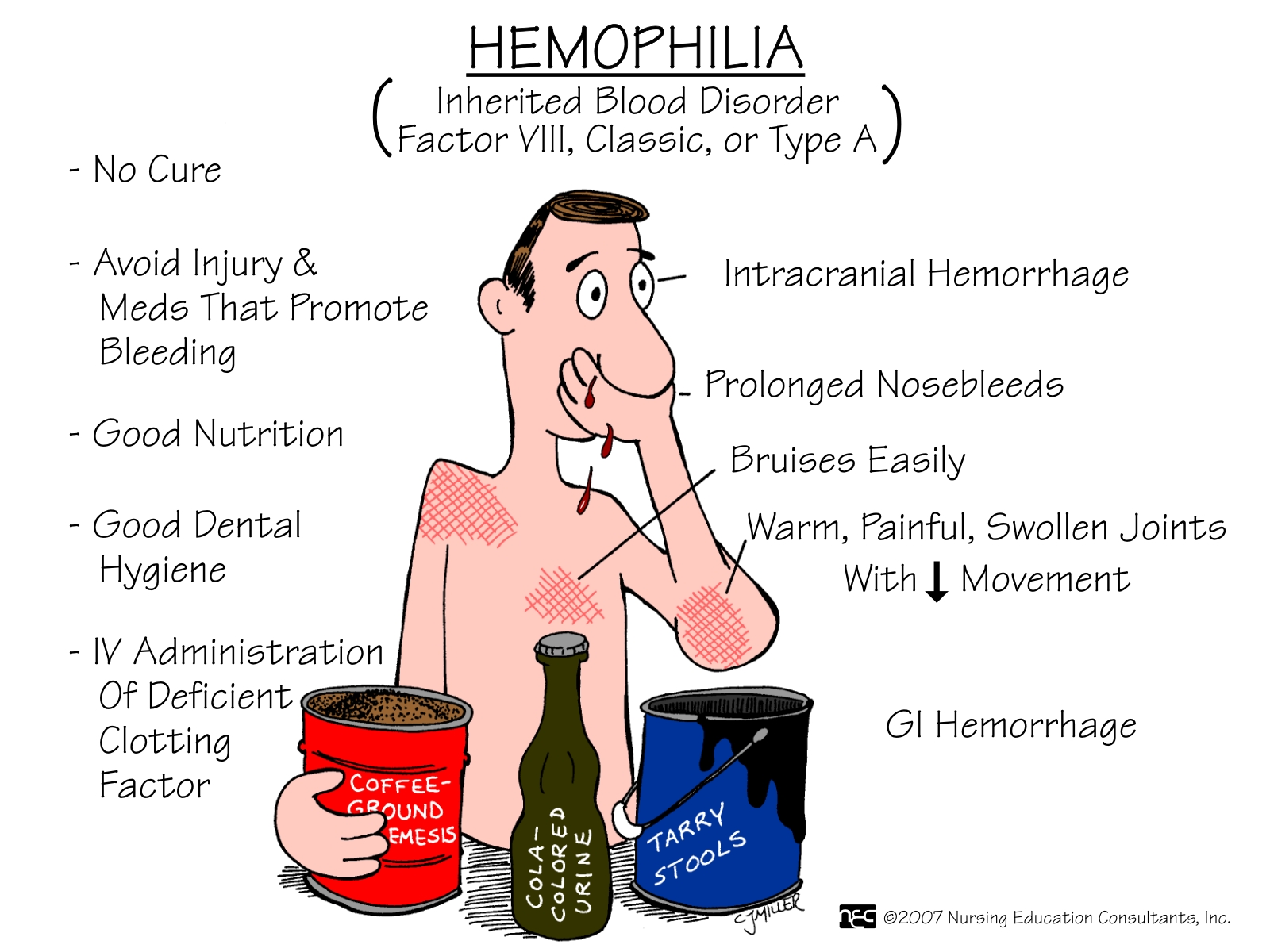 upper arm and forearm, the iliopsoas muscle (the front of the groin area), the thigh, and the calf. The joints that are most often affected are the knee, ankle, and elbow.
upper arm and forearm, the iliopsoas muscle (the front of the groin area), the thigh, and the calf. The joints that are most often affected are the knee, ankle, and elbow.
 upper arm and forearm, the iliopsoas muscle (the front of the groin area), the thigh, and the calf. The joints that are most often affected are the knee, ankle, and elbow.
upper arm and forearm, the iliopsoas muscle (the front of the groin area), the thigh, and the calf. The joints that are most often affected are the knee, ankle, and elbow.
If bleeding occurs many times into the same joint, the joint can become damaged and painful.
 Repeated bleeding can cause other health problems like arthritis. This can make it difficult to walk or do simple activities. However, the joints of the hands are not usually affected in hemophilia (unlike some kinds of arthritis).
Repeated bleeding can cause other health problems like arthritis. This can make it difficult to walk or do simple activities. However, the joints of the hands are not usually affected in hemophilia (unlike some kinds of arthritis).
Hemophilia is diagnosed by taking a blood sample and measuring the level of factor activity in the blood. Hemophilia A is diagnosed by testing the level of factor VIII activity. Hemophilia B is diagnosed by measuring the level of factor IX activity.
If the mother is a known carrier of hemophilia, testing can be done before a baby is born. Prenatal diagnosis can be done at 9 to 11 weeks by chorionic villus sampling (CVS) or by fetal blood sampling at a later stage (18 or more weeks).
TREATMENT
- Factor concentrates are the treatment of choice for hemophilia.
- Cryoprecipitate is derived from blood and contains a moderately high concentration of clotting factor VIII (but not IX). It is effective for joint and muscle bleeds
- In fresh frozen plasma (FFP) the red cells have been removed, leaving the blood proteins including clotting factors VIII and IX.
WHY AN AWARENESS?
Unfortunately, there are many people in the world, who receive poor treatment or no treatment at all, for hemophilia and related bleeding disorders. According to the World Federation of Hemophilia, about 1 in every 1000 person has a bleeding disorder; many are left untreated. The aim of World Hemophilia Day is to raise awareness about hemophilia and increase the availability of treatments for this condition around the world.
As with many other awareness campaigns, a simple but effective slogan is used to help raise awareness for the issues concerned. The slogan for World Hemophilia Day is 'Close The Gap', which reflects the difference in treatments available to people living in different parts of the world. By working together, it is hoped that we can close the gap of hemophilia care around the world. 'Close The Gap', is an apt phrase; when blood clots, a 'gap' is closed which stops bleeding.




.jpg)